
Jonathan Cawley
Dublin City University, Ireland
Title: Investigating the surface glycosylation of CHO cells using a combination of novel lectins, fluorescence microscopy and flow cytometry
Biography
Biography: Jonathan Cawley
Abstract
Chinese hamster ovary (CHO) cells are extensively used for the production of biopharmaceutical products. In 1985 the first recombinant proteins (i.e., monoclonal antibodies, blood factors, growth hormones and cytokines etc.) are produced in CHO cells. Proteins produced in CHO frequently undergo post-translational modifications (PTM). The most common PTM is glycosylation which can alter the function, stability, immunogenicity and efficacy of the resulting protein. Changes to the CHO glycocalyx, carbohydrate coat surrounding the cell membrane, may be informative to changes on the product being produced. It is therefore of great interest to understand how the host cell changes in different culture conditions. Fluorescent microscopy and flow cytometry are employed to perform glycoanalysis on CHO DP-12 cells. Recombinant eukaryotic lectins, purified using immobilized metal affinity chromatography (IMAC), and commercial plant and fungal lectins are used to probe CHO cells in order to establish a lectin binding profile.
Lectins from non-plant sources are perfect glycan probes as they are generally nontoxic but they are also superior to other binding proteins such as antibodies whose specificities for carbohydrate targets are ill-defined. In this work a recombinant eukaryotic lectin, Agrocybe aegerita lectin 2 (AAL-2), was expressed, purified and characterised. It was then successfully used to probe the CHO DP-12 surface, along with other lectins, demonstrating that a novel lectin produced recombinantly is the optimal choice of probe for investigating the cell surface glycosylation of live cells.
Image
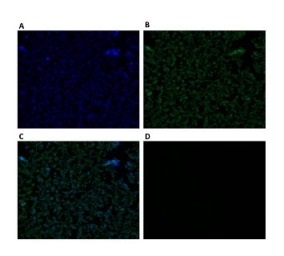
Figure 1: Fluorescent microscope images of CHO DP-12 cells probed with AAL-2. CHO DP- 12 cells were probed with the GlcNAc binding Agrocybe aegerita lectin 2 (AAL-2) for 30 min and viewed with a fluorescent microscope at 400x magnification.
A) Nuclear(Hoechst) stain. B) AAL-2:FITC stain. C) Merged image of A) and B). D) AAL-2:FITC stain + 200 mM GlcNAc - viewed in FITC filter.
References
1. O’Connor, B.F.,Monaghan, D. and Cawley, J. 2017. Lectin Affinity Chromatography (LAC). In: Walls, D and Loughran , S.T. eds. Protein Chromatography : Methods and Protocols. New York: Humana Press, pp. 411-420.
