
Nadine Legros
University of Münster, Germany
Title: Molecular lipid raft composition of primary human brain and kidney endothelial cells emphasizing Shiga toxin glycosphingolipid receptors
Biography
Biography: Nadine Legros
Abstract
Human brain and kidney endothelial cells are important targets for Shiga toxins (Stxs) produced by enterohemorrhagic Escherichia coli (EHEC) and play key roles in the pathogenesis of life-threatening extraintestinal complications. The clinically important Stx1a and Stx2a subtypes bind to glycosphingolipids (GSLs) of the globo-series. Primary endothelial cells are fastidious and sensible cells, which are eminently suitable as an optimal native cell type for analyzing Stx-mediated cellular injury. Lipid rafts represent supramolecular membrane microdomains that are enriched in certain types of lipids such as cholesterol, sphingomyelin and GSLs. Detergent-resistant membranes (DRMs), commonly used as lipid raft-analogous structures, represent the ruling method to assign association with lipid rafts.
Here we present novel data on the composition of lipid rafts prepared from primary human brain microvascular endothelial cells (pHBMECs) and primary human renal glomerular endothelial cells (pHRGECs). Most prominent receptor lipoforms of Stx-receptor GSLs of both types of endothelial cells were globotriaosylceramide (Galα4Galβ4Glcβ1Cer, Gb3Cer) and globotetraosylceramide (GalNAcβ3Galα4Galβ4Glcβ1Cer, Gb4Cer) with Cer (d18:1, C16:0), Cer (d18:1, C22:0) and Cer (d18:1, C24:1/C24:0), determined by electrospray ionization mass spectrometry in combination with thin-layer chromatography (TLC) immunochemical detection. Gb3Cer and Gb4Cer were found to co-distribute with canonical lipid raft-markers cholesterol and sphingomyelin as well as flotillin-2 in DRMs, which represent the liquid-ordered membrane phase and indicate their association with lipid rafts. Figure 1 exemplarily shows the DRM and nonDRM distribution of Gb3Cer and Gb4Cer detected in sucrose density gradient fractions of pHBMECs using Stx1a- and Stx2a-TLC overlay assays. On the other hand, lyso-phosphatidylcholine was identified as a nonDRM marker phospholipid of the liquid-disordered membrane phase. Increasing knowledge on membranes of brain and kidney endothelial cells might help to develop new therapeutic strategies to fight EHEC infections.
Image
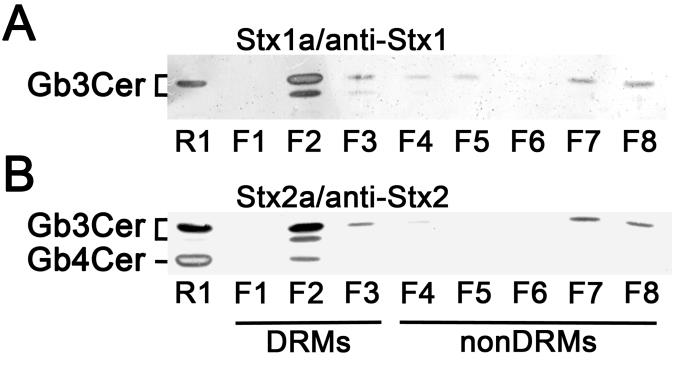
Fig. 1. Detection of Stx-binding GSLs in sucrose density gradient fractions of pHBMECs. (A) TLC overlay assay detection of Stx1a- and (B) Stx2a-binding GSLs using Stx1- and Stx2-specific antibody, respectively. GSL extracts correspond to 8 × 105 cells and 2 µg of reference neutral GSLs from human erythrocytes (R1) were applied in each assay (Legros et al. 2017a).
References
1. Bauwens A, Betz J, Meisen I, Kemper B, Karch H, Müthing J (2013) Facing glycosphingolipid-Shiga toxin interaction: dire straits for endothelial cells of the human vasculature. Cell. Mol. Life Sci. 70: 425-457.
2. Lee MS, Koo S, Tesh VL (2016) Shiga toxins as multi-functional proteins: induction of host cellular stress responses, role in pathogenesis and therapeutic applications. Toxins (Basel) 8:pii: E77.
3. Legros N, Dusny S, Humpf HU, Pohlentz G, Karch H, Müthing J (2017a) Shiga toxin glycosphingolipid receptors and their lipid membrane ensemble in primary human blood–brain barrier endothelial cells.
4. Legros N, Pohlentz G, Runde J, Dusny S, Humpf HU, Karch H, Müthing J (2017b) D-PDMP-induced changes of membrane microdomain lipids of primary human renal glomerular endothelial cells with focus on Shiga toxin glycosphingolipid receptors (submitted).
5. Obrig TG, Karpman D (2012) Shiga toxin pathogenesis: kidney complications and renal failure. Curr. Top. Microbiol. Immunol. 357: 105-136.
6. Trachtman H, Austin C, Lewinski M, Stahl RA (2012). Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat. Rev. Nephrol. 8: 658-669.
