
Sven Bahrke
Glycotope GmbH, Germany
Title: Combination of high resolution HILIC-UPLC-FLD-ESI-QTOF MS/MS with automated data processing for structure determination and quantification of proteins with complex glycan patterns
Biography
Biography: Sven Bahrke
Abstract
Optimal functionality and limitation of side effects of recombinant therapeutic proteins require a glycosylation profile that mimics the complexity of the natural human glycosylation as much as possible. As a result, the need for characterization of complex glycosylation patterns in R&D and regulatory environments is evident. The coupling of high resolution chromatography and mass spectrometry is the method of choice for analysis of both simple and complex glycan patterns. However, LC-MS/MS data of glycans are highly complex. Thus, manual data processing and evaluation are time consuming as well as error-prone. We developed an automated method to cope with intense glycoanalysis requirements in biopharmaceutical characterization.
We applied MS-sensitive fluorescence labeling of liberated glycans from biopharmaceuticals and high resolution chromatography with fluorescence detection in line coupled to tandem mass spectrometry (HILIC-UPLC-FLD-ESI-QTOF MS/MS). This approach facilitates the unambiguous identification and reasonable quantification of biologically important N-glycan parameters like antennarity, sialic acid, bisecting N-acetylglucosamine, core or outer-arm fucose and glycan sulfation optionally in GxP quality. We developed software that automatically provides such parameters and minimizes user intervention.
Automated Bruker QTOF (Compact / Impact II) data evaluation using script based software allowed an evolution of the automated analysis, which facilitated the hybridization of fluorescence and MS data and empirical MS/MS spectra library matching for automatic structure identification and fluorescence based quantification of glycan structures.
The feasibility of the method was successfully proven for biopharmaceuticals resulting from Glycotope’s human cell lines for production of recombinant proteins with complex glycosylation with improvements in sialylation, galactosylation, fucosylation, antennarity which results in higher bioactivity, stability, serum half-life time and reduced immunogenicity. Even for glycoproteins comprising over 60 individual glycan structures, the complete identification and quantification starting from approximately 10µg of glycoprotein is available within one day in an automated workflow.
Image
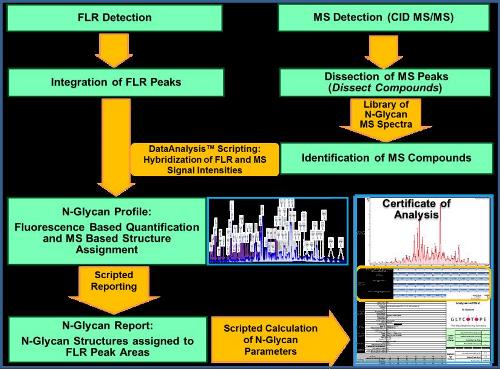
Figure 1. Workflow for the automated processing of LC-MS/MS data for quantitative analysis of the human-like N-glycosylation of glycoproteins produced by the GlycoExpress™ cell platform
