
Andras Guttman
University of Debrecen, Hungary
Title: Analytical glycomics: Biopharmaceutical applications
Biography
Biography: Andras Guttman
Abstract
Full characterization of the N-glycosylation moieties of biopharmaceuticals is of high importance, especially when glycovariants may impact the biological effect. Well over half of the new generation protein therapeutics are monoclonal antibodies, in which the attached oligosaccharides not only affect their physicochemical properties and stability, but also their receptor binding activity, circulating half-life and last but not least, their immunogenicity. Therefore, high performance glycoanalytical techniques are of great demand for N-glycosylation analysis of therapeutic antibodies, especially during clone selection, process development and lot release. Analysis of complex carbohydrates is a very challenging task due to the lack of their chromophore / fluorophore activity and, in many instances, easily ionizable groups, necessitating derivatization before electric field mediated analysis. Full N-glycosylation characterization may also require sequencing with consecutive exoglycosidase digestion steps, followed by capillary electrophoresis analysis. In this presentation the state of the art of liquid phase separation methods will be conferred for comprehensive structural elucidation of protein N-glycosylation, mostly using capillary electrophoresis and its combination with mass spectrometry (CESI-MS). Assisted by the emerging field of glycoinformatics, assignment of the identity of the separated glycan structures will be demonstrated by using the recently introduced GUcal software.
Image
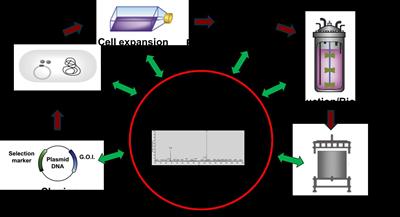
Figure 1. Glycoanalytical needs during the manufacturing process of recombinant therapeutic proteins
References
1. M.Szigeti, C.Lew, K.Roby, A.Guttman, Fully automated sample preparation with ultrafast N-glycosylation analysis of therapeutic antibodies, JALA 21 (2016) 281-286
2. A.Szekrenyes, S.S.Park, M.Santos, B.Warren, A.Jones, E.Cosgrave, T.Haxo, S.Pourkaveh, Z.Szabo, Z.Sosic, P.Feng, Cs.Varadi, JB.Falmagne, P.Sejwal, D.Michels, G.Freckleton, M.Hamm, AManuilov, T.Duffy, M.Schwartz, JK.Luo, J.van Dyck, PK.Leung, M.Olajos, B.Moritz, R.Kowle, G.Kai, W.Wenbo, J.Wegstein, A.Guttman, Multi-Site N-Glycan Mapping Study 1: CE-LIF, mAbs 8 (2016) 56-64.
3. A.Guttman, M.Kerekgyarto, G.Jarvas, The effect of separation temperature on structure specific glycan migration in capillary electrophoresis, Anal.Chem 87 (2015) 11630-11634.
4. C.Lew, JL.Gallegos-Perez, B.Fonslow, A.Guttman, Rapid level-3 characterization of therapeutic antibodies by capillary electrophoresis electrospray ionization mass spectrometry (CESI-MS), J.Chrom.Sci. 53 (2015) 443-449.
