Day 1 :
Keynote Forum
Julian M Menter
Morehouse School of Medicine, USA
Keynote: The interaction between dermal type I collagen and hyaluran (HA) in phosphate buffered solution, pH = 7.4.
Time : 09:10-09:40

Biography:
Dr. Menter received his PhD degree in Chemistry from the George Washington University in 1969. He completed a postdoctoral fellowship with Prof. Dr. Theodor Foerster at the Institut fuer physikalische Chemie der Universtiaet Stuttgart, Germany. Subsequently, he was at the University of Alabama, Birmingham, and the VA Medical Center (Atlanta) He currently serves as Research Professor of Biochemistry at Morehouse School of Medicine. Dr. Menter is recognized internationally for his work in the areas of collagen photochemistry and melanin photobiology as pertaining to redox reactivity
Abstract:
As dermal collagen fluorescence spectra are time – and environment – sensitive, they show promise as biomarkers and prognosticators of damage due to aging and other pathology in general. The rate of photochemical formation of dityrosine form internal tyrosine residues is quasi – linear, and its slope can serve as an indicator of the rate of ground and excited state molecular damage. In Vivo dermal collagen is embedded in in surrounding extra cellular matrix (ECM) containing a complex of hyaluronic acid (HA) and proteoglycan (PG).In this work, we report preliminary results of Collagen – HA interactions in an model in vitro system. Methodology: Solutions containing 1.0 mg/ml type I collagen + 2.0 mg/ml HA (Elastin Products) in 0.1 M phosphate buffer, pH 7.4 were irradiated from 0 – 200 min. in a thermostatted cuvette (Hellma Cells) with a 4 W filtered UVG – 11 hand lamp emitting at 254 nm. Dityrosine formation as a function of time was monitored by its fluorescence at excitation/emission wavelengths 325/400 nm for temperatures between 13 – 60 oC. Results and Discussion: For T < Tm (~ 36 oC) HA retards the rate of dityrosine formation by ~ 20 – 30 %, indicating stabilization of collagen scaffolding by HA. At T > Tm, where the coiled conformation dominates, there appears to be no systematic effect of HA on collagen stability. Thus, stabilization of collagen helical structure seems to be one important function of the ECM.
References
1. J. M. Menter, G. D. Williamson, C. L. Moore, and I. Willis (1994). Melanin as an Electron Transfer Reagent. in "Melanin: Its Role in Human Photoprotection" Publishing Co. Overland Park, KS; pp. 23-29.
2. JM Menter, AM Patta, RM Sayre, J Dowdy, and I Willis (2001). Effect of UV irradiation on Type I Collagen Fibril Formation in Neutral Collagen Solutions. Photodermatol. Photoimmunol. Photomed. 17:114-120.
3. J.M.Menter, L.M. Cornelison, L Cannick, A.M. Patta, J.C.Dowdy, R.M. Sayre, I.K. Abukhalaf, N.S. Silvestrov, and I. Willis. (2003) Effect of UV on the Susceptibility of Acid – Soluble Skh – 1 Hairless Mouse Collagen to Collagenase. Photochem. Photoimmunol. Photomed: 19 : 28 – 34
4. J. M. Menter (2006). Temperature Dependence of Collagen Fluorescence. Photochemical and Photobiological Sciences 5: 403 – 410.
5. J.M. Menter, I.K. Abukhalaf , A.M. Patta, N.A. Silvestrov, and I Willis (2007). Fluorescence of Putative Chromophores in Skh – 1 and Citrate – Soluble Calf Skin Collagens. Photochem. Photoimmunol. Photomed: 23 : 222 – 228.
Keynote Forum
Myron R Szewczuk
Queen’s University, Canada
Keynote: Specific sialoglycan structures on the cell surface correlate with the ability of cancer cells to form avascular multicellular 3D tumor spheroids and in vivo xenograft tumors
Time : 09:40-10:10

Biography:
Dr. Szewczuk is Full Professor of Immunology and Medicine, Queen’s University, Kingston, Ontario Canada. Dr. Szewczuk’s current research is focusing on the role of glycosylation in receptor activation with a particular focus on alternate new active tumor targeting drug delivery systems.
Abstract:
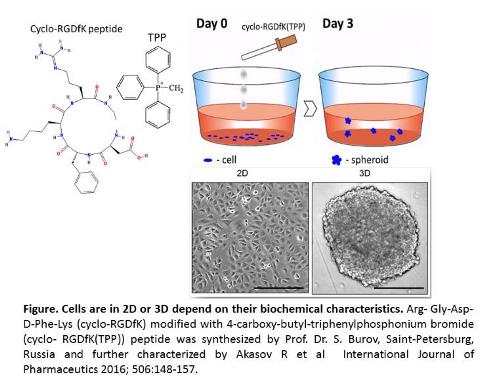
Multicellular 3D tumor spheroid (MTS) formation in cancer research has been designed to mimic tumor-like developmental patterns in vitro. Tumor growth and invasion is known to be highly influenced by aberrant cell surface-specific sialoglycans on cell surface glycoproteins. Aberrant sialoglycan patterns that facilitate MTS formation has not been well defined. To evaluate the role of sialylation of cancer cell surfaces in spheroid formation, we used the cyclo-RGDfK(TPP) approach to biochemically induce cell aggregation and compaction, transmogrifying monolayer cancer cells into tumor spheroids. The cyclo-RGDfK(TPP) peptide-based platform causes specific biochemical alterations of cell surface receptors inducing self-assembly in monolayer cell cultures into 3D MTS by facilitating cell-cell recognitions, interactions and adhesion. Matrix-free spheroids from breast MCF-7 and pancreatic PANC1 cancer cell lines and their respective tamoxifen (TMX) and gemcitabine (Gem) resistant variants formed tight spheroids while all PANC1 cells formed loose aggregates. MCF-7 and PANC1 cells and their drug-resistant variants expressed different sialic acid (SA) content on their cell surfaces. α-2,3- and α-2,6-sialic acid surface residues facilitated spheroid formation under cyclo-RGDfK(TPP)-induced self-assembly. Pretreatment with α-2,3-SA specific Maackia amurensis (MAL-II) lectin, α-2,6-SA specific Sambucus nigra (SNA) lectin, and exogenous α-2,6-SA specific neuraminidase (Vibrio cholerae) dose dependently reduced spheroid volume. Oseltamivir phosphate (OP) treatment enhanced cell aggregation and compaction forming spheroids. PANC1 and MDA-MB231 xenograft tumors from untreated and OP-treated RAGxCγ double mutant mice expressed significantly higher levels of α-2,3-SA over a-2,6-SA. The present report provides evidence for the important role of specific sialoglycan structures expressed on cancer cells to form avascular multicellular tumor spheroids and in vivo xenograft tumors. Future studies should build upon these findings and explore alternate and novel methods to target the cancer cell glycome and the unique sialylation patterns of the adhesion molecules involved in spheroid formation and tumor progression.
Image
Networking & Refreshment Break 10:15-10:30 @ Foyer
